Anode and Cathode in Electrolysis
They need to gain enough electrons to make them neutral. Cations go to the cathode.

Electrolysis Process On Passing Electric Current The Cations Move Towards The Cathode And Get Deposited Piscinas De Agua Salada Escuela De Natacion Piscinas
A solid oxide fuel cell or SOFC is an electrochemical conversion device that produces electricity directly from oxidizing a fuel.

. Most electrolysis problems are really stoichiometry problems with the addition of an amount of electric current. The anode is a positively charged electrode. This definition can be recalled by using the mnemonic CCD for Cathode Current DepartsA conventional current describes the direction in which positive charges move.
The cathode is made of pure copper or a support metal such as stainless steel. In the electrolysis process electrolyzers use electricity for water-splitting. 2Br- Br 2 2e-.
Different electrolyzers function in different ways mainly due to the different type of electrolyte. The two common terms we hear is cathode and anode. Explain corrosion as an electrochemical process.
The less noble metal in a reaction will be the anode. Electrorefining of copper was first demonstrated experimentally by Maximilian Duke of Leuchtenberg in 1847. Advantages of this class of fuel cells include high combined heat and power efficiency long-term stability fuel flexibility low emissions and.
The cathode is the current that leaves the electrodes or cathode is a result of reduction reaction taking place in an electrolyte mixture. According to the equations for the two half-reactions the indicator should turn yellow at the anode and blue at the cathode. The entire process of electrolysis is carried out in an electrolytic cell.
Fuel cells are characterized by their electrolyte material. Commercially electrolytic cells are used in electrorefining and electrowinning of several non-ferrous metals. The process of electrolysis sees electrons being stripped from the anode.
The more noble metal in the reaction will always be the cathode. Electrolysis is a technique that uses a direct electric current DC. This contrasts with a cathode an electrode of the device through which conventional current leaves the deviceA common mnemonic is ACID for anode current into device.
Releasing electrons to the anode. This reduces the effective area for current and increases the local current density. Electroplating and electrolysis welding cathodic protection.
The direction of conventional current the flow of positive charges in a circuit is. Thus the standard potential of the water electrolysis cell E o cell E o cathode E o anode is -1229 V at 25 C at pH 0 H 10 MAt 25 C with pH 7 H 10 10 7 M the potential is unchanged based on the Nernst equationHowever calculations regarding individual electrode equilibrium potentials requires some corrections taking into account the activity coefficients. An example is the electrolysis of an aqueous sodium chloride solutionalthough oxygen should be produced at the anode.
The reaction takes place in a unit called an electrolyzer. Like fuel cells electrolyzers consist of an anode and a cathode separated by an electrolyte. Here electrons are released from the electrode and the surrounding solution is reduced.
Is now an accepted convention that we keep the anode on the left and the cathode on the right while representing the galvanic cell. Then they head towards the cathode. Electrowinning is the oldest industrial electrolytic process.
So what does that mean. A cathode is a negatively charged electrode. E As per electrolytic reactions 4H 1 are needed at cathode and 4OH-at the anode and two molecules of water are produced at the anode.
An electrochemical reaction occurs which is 7080 efficient and is the most established well-known commercially available technology for water-splitting. According to the general definition an electrode is a substance that helps in electricity conduction wherein the electric current either leaves or enters the non-metallic medium such as an electrolytic cell. 2 H 2 O O 2 4 H 4 e-Faradays Law.
Many devices have other electrodes to control operation eg base gate control grid. The electrons enter the device through the cathode and exit the device through the anode. A cathode is the electrode from which a conventional current leaves a polarized electrical device.
Salt water chlorination is a process that uses dissolved salt 100036000 ppm or 136 gL for the chlorination of swimming pools and hot tubsThe chlorine generator also known as salt cell salt generator salt chlorinator or SWG uses electrolysis in the presence of dissolved salt to produce chlorine gas or its dissolved forms hypochlorous acid and sodium hypochlorite which. A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a nearly constant volume independent of pressure. Without the negatively charged coating the anode only works for around 12 hours in seawater according to Michael Kenney a graduate student in the Dai lab and co-lead author on the paper.
The ions are forced to undergo either oxidation at the anode or reduction at the cathode. Linear paired glycerol valorization at the NiSe 2 cathode in O 2-saturated 01 or 05 M NaHSO 4 Na 2 SO 4 solution containing 50 mM glycerol and 05 mM Fe 2 pH 28 and at the PtC anode in. This is called oxidationFor example.
Electrolysis is the process of using electricity to split water into hydrogen and oxygen. The anode consists of an unrefined sample of the metal. Describe the construction of some primary and secondary batteries and fuel cells.
Before we learn about the terms cathode and anode it is important to understand what an electrode is. So an Al 3 ion needs to gain. And in order to separate the different gases formed at the cathode and anode respectively the use of a.
2 H 2 O 2 e-H 2 2 OH-Anode. The electrolysis can be done using two weighed copper strips. This migration takes place in a liquid medium that.
Likewise the cathode reduces sodium ions Na which accept electrons from the cathode and deposits on the cathode as sodium. Hence for every two molecules of water two molecules of hydrogen and one molecule of oxygen are liberated at the cathode and anode respectively. In this cell ions will migrate to or away from the anode or cathode.
James Elkington patented the commercial process. This is to confirm that the mass gained at the cathode is. Electrons have a negative electrical charge so the movement of electrons is opposite to that of the conventional.
Electrolysis is used to drive an oxidation-reduction reaction in a direction in which it does not occur spontaneously. As such it is one of the four fundamental states of matter the others being solid gas and plasma and is the only state with a definite volume but no fixed shapeA liquid is made up of tiny vibrating particles of matter such as. Electrolysis involves passing an electric current through either a molten salt or an ionic solution.
In a vacuum tube or a semiconductor having polarity diodes electrolytic capacitors the anode is the positive electrode and the cathode the negative. Bubble overpotential is a specific form of concentration overpotential and is due to the evolution of gas at either the anode or cathode. While in the HTSE process water is first converted to steam by using nuclear thermal energy rather than electricity and then dissociated at the cathode to form the hydrogen molecules as well as oxygen ions which subsequently migrate through the solid oxide electrolyte material and then form oxygen molecules at the anode surface.
The SOFC has a solid oxide or ceramic electrolyte. F This is because HNO 3 is volatile. The English chemist Humphry Davy obtained sodium metal in elemental form for the first time in 1807 by the electrolysis of molten sodium hydroxide.
An anode is an electrode of a polarized electrical device through which conventional current enters the device.

Physical Chemistry Positive Or Negative Anode Cathode In Electrolytic Galvanic Cell Chemistry Stack Electrochemistry Chemistry Classroom Teaching Chemistry

Look4chemistry Electrolytic Cell Electrochemistry Cell Positive And Negative

Electrolytic Cell Electrolysis Of Nacl Chemie

Electrolysis Of Copper Sulfate Solution With Impure Copper Anode And Pure Copper Cathode Copper Purification Technology Educat Pure Products Copper Solutions

Electrolysis Chemical Changes Electrochemistry Energy
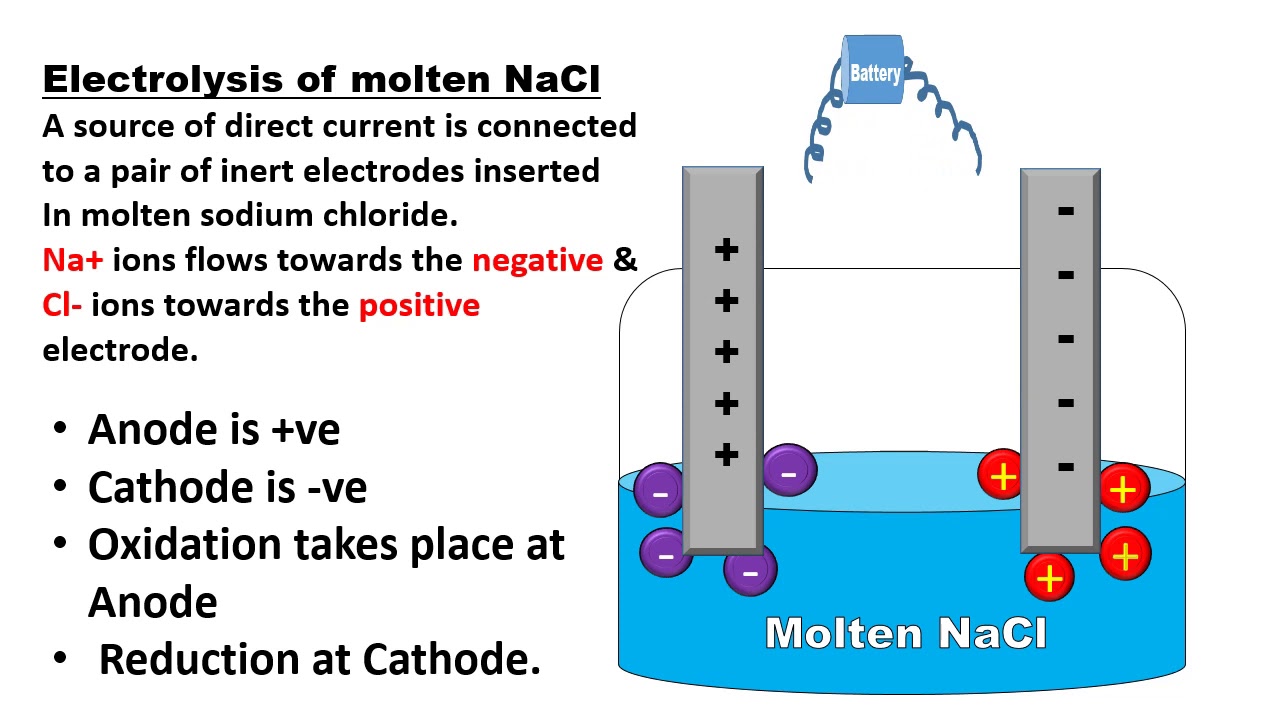
What Is Electrolytic Cell Electrochemistry Chemistry Basics Chemistry
0 Response to "Anode and Cathode in Electrolysis"
Post a Comment